electronic configuration of iron|What is the electron configuration of iron? : Pilipinas Learn how to write the electron configuration for iron and its ions using the period table or an electron configuration chart. See the video, examples and step-by-step tutorial for writing the electron configurations. Capellan Institute of Technology. San Pablo City, Laguna menu ≡. Overview. Courses. Certificate programs. Computer Technology courses offered at CIT. Certificate courses; Computer Hardware Servicing NCII Disclaimer: The data provided in this page was collected from internet sources as well as by calling or emailing the school's .
electronic configuration of iron,Learn how to write the electron configuration for iron and its ions using the period table or an electron configuration chart. See the video, examples and step-by-step tutorial for writing the electron configurations.
Learn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, .Learn how to write the electronic configuration of iron and its oxidation states, valency, and applications. Find out the reduced form of iron and the difference between iron and iron(II) and iron(III). Learn how to write the electron configuration of iron through orbitals or orbitals using Bohr model or Aufbau principle. See the electron holding capacity, subshells, and orbitals of iron and its ions.What is the electron configuration of iron? Learn how to write the electronic configuration of iron and its ions, and the valency of iron in different oxidation states. Find out the properties, allotropic forms and .
Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the corrected diagram. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. How many valence electrons does Iron have? The electron configuration .
Learn how to write the electronic configuration of iron, its valency, oxidation states, properties and applications. Find out the formula of rust, the role of iron in .Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to .
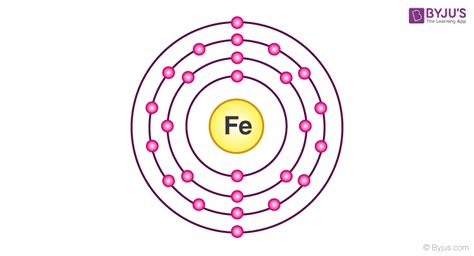
Schematic electronic configuration of iron. The Kossel shell structure of iron. Atomic spectrum. A representation of the atomic spectrum of iron. Ionisation Energies and electron affinity. The .
Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.
Electronic Iron configuration will be : \[1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2} 3d^{6}\]. Iron, a chemical substance or element, is a metal generally represented by its symbol Fe. Its symbol is short for Ferrum which is a Latin term. The atomic number of iron is 26. Iron is a member of group 8 and the first transition series of the periodic .
Aufbau Principle. Aufbau comes from the German "structure" or building up and Aufbauprinzip, is the "building up principle" where we assign electrons to the lowest energy orbitals first, and then successively add them to .

The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.electronic configuration of ironElectron configuration 3d 6 4s 2: Electrons per shell: 2, 8, 14, 2: Physical properties; . Due to its electronic structure, iron has a very large coordination and organometallic chemistry. The two enantiomorphs of the ferrioxalate ion. Many coordination compounds of iron are known.

The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 .For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence . Write the electronic configurations of: neutral iron, iron(II) ion, and; iron(III) ion. Answer. The atomic number of iron is 26 so there are 26 protons in the species. Fe: [Ar . 88 th ed. Sct. 1, Prt. 1 Electron Configuration and Ionization Energy of Neutral Atoms in the Ground State; 13-14. CRC Handbook, 88 th ed. Sct. 4, Prt. 1 .
The electronic configuration of iron will be reduced to [Ar]3d6. And the Oxidation state of iron becomes +2. Iron will attain more stability by the loss of one more electron from the d subshell and thereby changing the electronic configuration to [Ar]3d5. This has more stability than iron in the +2-oxidation state and is because of the reason . If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and .
Electronic Configuration of Iron. The shorthand electronic configuration of iron (Fe), which has an atomic number of 26, is \( [Ar] 3d^6 4s^2 \). Its electronic configuration can be written as \( 1s^2 2s^2 .In the case of Iron, its electron configuration is written as 1s2 2s2 2p6 3s2 3p6 3d6 4s2, which corresponds to the filling of the different orbitals as shown in the orbital diagram. Additionally, the presence of unpaired .
Here are the changes in the electronic structure of iron to make the 2+ or the 3+ ion. Fe [Ar] 3d 6 4s 2: Fe 2 + [Ar] 3d 6: Fe 3 + [Ar] 3d 5: The 4s orbital and the 3d orbitals have very similar energies. There is not a huge jump in the amount of energy you need to remove the third electron compared with the first and second. Iron is element 26 in the periodic table. Its electron configuration is 1s² 2s²2p⁶ 3s²3p⁶ 4s²3d⁶ or [Ar]4s²3d⁶.
Example \(\PageIndex{1}\): Iron. Consider the electronic structure of neutral iron and iron (III). To write the electronic structure for Fe 3 +: Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2; . The Full Story of the Electron Configurations of the Transition Elements: Journal of Chemical Education, Vol. 87 No. 4 April 2010. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 To write an electron configuration for any element you'll need to apply the Aufbau principle and use a diagonal diagram. Both are explained in the video below. Of the elements discussed in the video, iron is most like Sc, but iron has 5 more electrons in the last subshell which is filled with electrons making its .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.
electronic configuration of iron|What is the electron configuration of iron?
PH0 · What is the electron configuration of iron?
PH1 · WebElements Periodic Table » Iron » properties of
PH2 · Iron Electron Configuration (Fe) with Orbital Diagram
PH3 · Iron
PH4 · Electronic Configuration of Iron: Fe element
PH5 · Electronic Configuration of Iron
PH6 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH7 · Electron Configuration for Iron (Fe and Fe2+, Fe3